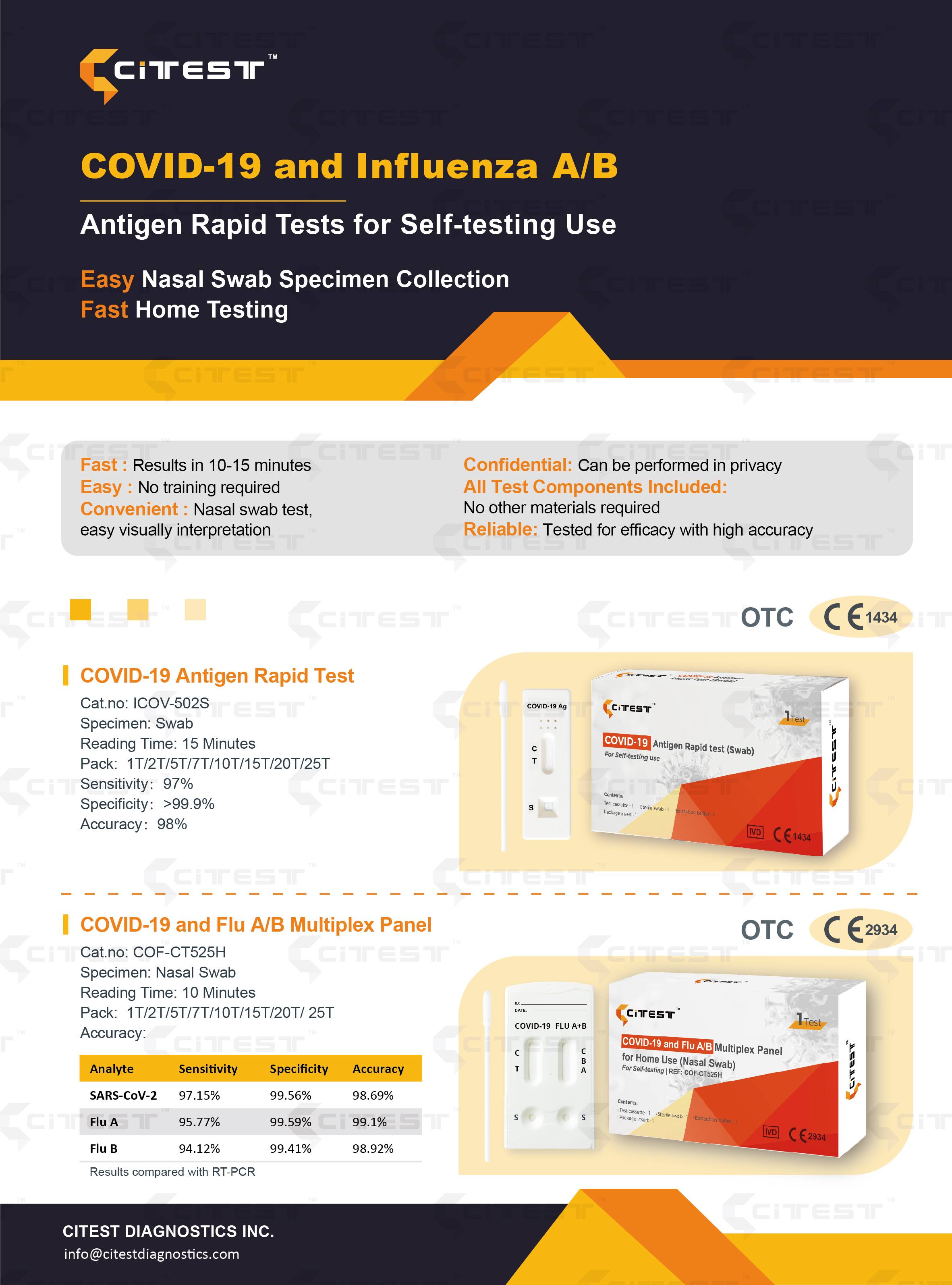
Covid-19 and Influenza A/B - Antigen Rapid Test for Self-testing Use
On March 11, 2020, the World Health Organization (WHO) declared COVID-19, the disease caused by the SARS-CoV-2, a pandemic. Now, nearly two years later, COVID-19 is still classified as a global pandemic with cases continuing to rise. The novel coronavirus can cause severe complications including acute respiratory distress syndrome, acute myocardial disease, and metabolic acidosis, which can cause irreversible damage, and even lead to death. COVID-19 is transmitted between humans and has spread rapidly
COVID-19 Antigen Rapid Test for Self Testing (CE1434 OTC) (Nasal Swab)
The COVID-19 Antigen Rapid Test (Nasal Swab) is a single-use test kit intended to detect the SARS-CoV-2 that causes COVID-19 with self-collected nasal swab specimen from symptomatic individuals who are suspected of being infected with COVID-19. This test is designed for home use with self-collected nasal swab samples in individuals who are suspected of COVID-19. The COVID-19 Antigen Rapid Test (Nasal Swab) obtain a preliminary results only, the final confirmation should be based on clinical diagnostic results.
COVID-19 Antigen Rapid Test for Self Testing (CE1434 OTC) (Oral fluid)
The COVID-19 Antigen Rapid Test (Oral Fluid) is a single-use test kit intended to detect the novel coronavirus SARS-CoV-2 that causes COVID-19 in human oral fluid. This test is designed for home use with self-collected oral fluid samples in individuals who are suspected of COVID-19. The COVID-19 Antigen Rapid Test (Oral Fluid) obtain a preliminary results only, the final confirmation should be based on clinical diagnostic results.